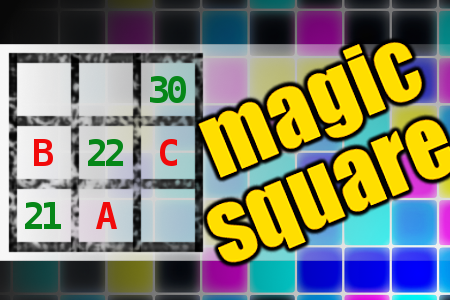
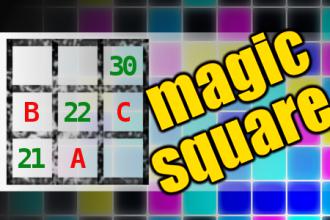
MAGIC SQUARE: Calculate A+B*C
The aim is to place the some numbers from the list (2, 3, 5, 6, 11, 14, 18, 21, 22, 30, 73, 93) into the empty squares and squares marked with A, B an C. Sum of each row and column should be equal. All the numbers of the magic square must be different. Find values for A, B, and C. Solution is A+B*C.Correct answers: 38
The first user who solved this task is Kristina Ko.
#brainteasers #math #magicsquare