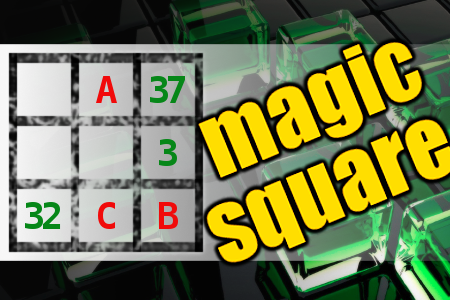
MAGIC SQUARE: Calculate A*B*C
The aim is to place the some numbers from the list (1, 3, 8, 14, 16, 21, 30, 32, 37, 38, 82) into the empty squares and squares marked with A, B an C. Sum of each row and column should be equal. All the numbers of the magic square must be different. Find values for A, B, and C. Solution is A*B*C.Correct answers: 27
The first user who solved this task is Linda Tate Young.
#brainteasers #math #magicsquare

Few classic Dad Jokes, and few very fresh
What do sprinters eat before a race?
Nothing, they fast.
I tell dad jokes all the time even though I’m not actually a dad
I’m a faux pa.
I changed all my passwords to 'Kenny'
Now I have all Kenny Loggins
What did the duck say when he bought the chap-stick?
Put it on my bill.
I dreamt last night that I was a muffler...
I woke up exhausted.
A friend had a new baby girl. Her coworker asked: “What’s her name?”
My friend replied: "Melanie Noelle."
Her coworker: "How do you spell it, then?"
I spent all my money collecting every bird species in my zoo, except one. My wife hates it.
But I have no egrets.