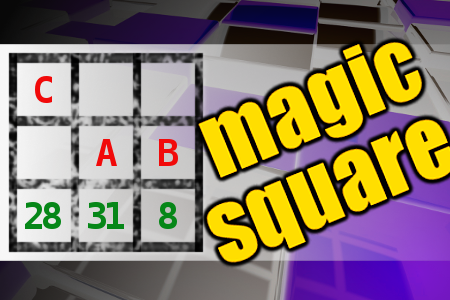
MAGIC SQUARE: Calculate A+B+C
The aim is to place the some numbers from the list (7, 8, 17, 19, 21, 22, 28, 29, 31, 38) into the empty squares and squares marked with A, B an C. Sum of each row and column should be equal. All the numbers of the magic square must be different. Find values for A, B, and C. Solution is A+B+C.Correct answers: 2
#brainteasers #math #magicsquare