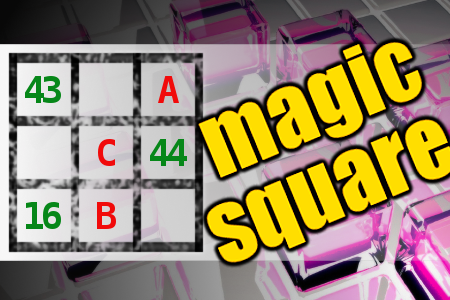
MAGIC SQUARE: Calculate A+B+C
The aim is to place the some numbers from the list (6, 7, 8, 9, 15, 16, 17, 20, 43, 44, 45, 90) into the empty squares and squares marked with A, B an C. Sum of each row and column should be equal. All the numbers of the magic square must be different. Find values for A, B, and C. Solution is A+B+C.
Duck Hunting
A city slicker shoots a duck out in the country. As he's retrieving it, a farmer walks up and stops him, claiming that since the duck is on his farm, it technically belongs to him. After minutes of arguing, the farmer proposes they settle the matter "country style."
"What's country style?" asks the city boy.
"Out here in the country," the farmer says: "when two fellers have a dispute, one feller kicks the other one in the balls as hard as he can. Then that feller, why, he kicks the first one as hard as he can. And so forth. Last man standin' wins the dispute."
Warily the city boy agrees and prepares himself. The farmer hauls off and kicks him in the groin with all his might. The city boy falls to the ground in the most intense pain he's ever felt, crying like a baby and rolling around on the ground. Finally he staggers to his feet and says: "All right, n-now it's–it's m-my turn."
The farmer grins: "Forget it, you win. Keep the duck."