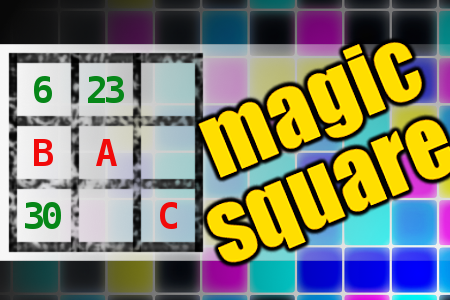
MAGIC SQUARE: Calculate A*B+C
The aim is to place the some numbers from the list (5, 6, 15, 23, 24, 30, 31, 33, 40, 47) into the empty squares and squares marked with A, B an C. Sum of each row and column should be equal. All the numbers of the magic square must be different. Find values for A, B, and C. Solution is A*B+C.Correct answers: 4
#brainteasers #math #magicsquare

A little boy and his grandfath...
A little boy and his grandfather are raking leaves in the yard. Thelittle boy finds an earthworm trying to get back into its hole. He says,"Grandpa, I bet I can put that worm back in that hole." The grandfatherreplies, "I'll bet you five dollars you can't. It's too wiggly and limpto put back in that little hole."
The little boy runs into the house and comes back out with a can ofhairspray. He sprays the worm until it is straight and stiff as a board.Then he puts the worm back into the hole.
The grandfather hands the little boy five dollars, grabs the hairspray,and runs into the house. Thirty minutes later the grandfather comes backout and hands the little boy another five dollars. The little boy says,
"Grandpa, you already gave me five dollars."
The grandfather replies, "I know. That's from your grandma
The little boy runs into the house and comes back out with a can ofhairspray. He sprays the worm until it is straight and stiff as a board.Then he puts the worm back into the hole.
The grandfather hands the little boy five dollars, grabs the hairspray,and runs into the house. Thirty minutes later the grandfather comes backout and hands the little boy another five dollars. The little boy says,
"Grandpa, you already gave me five dollars."
The grandfather replies, "I know. That's from your grandma