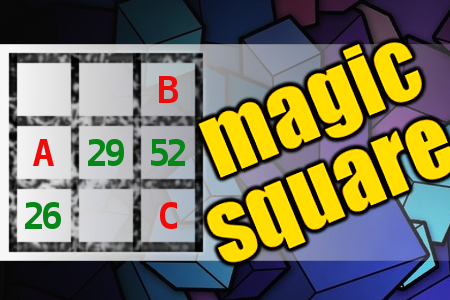
MAGIC SQUARE: Calculate A+B-C
The aim is to place the some numbers from the list (7, 8, 11, 25, 26, 29, 51, 52, 55) into the empty squares and squares marked with A, B an C. Sum of each row and column should be equal. All the numbers of the magic square must be different. Find values for A, B, and C. Solution is A+B-C.Correct answers: 2
#brainteasers #math #magicsquare

At school one morning the teac...
At school one morning the teacher asked little Johnny what he had for breakfast. Little Johnny said, well, on my way to school I come cross this Apple tree, so I climbed up there and started eating apples. I guess I eat about six, said little Johnny. No, said the teacher, it’s ate! Little Johnny said well it could've been eight I don't remember.