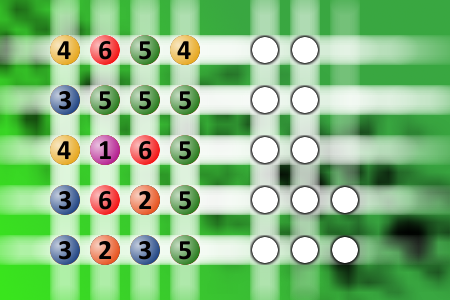
What a winning combination?
The computer chose a secret code (sequence of 4 digits from 1 to 6). Your goal is to find that code. Black circles indicate the number of hits on the right spot. White circles indicate the number of hits on the wrong spot.
Children Are Quick
TEACHER: Why are you late?
STUDENT: Class started before I got here.
____________________________________
TEACHER: John, why are you doing your math multiplication on the floor?
JOHN: You told me to do it without using tables.
____________________________________
TEACHER: Glenn, how do you spell 'crocodile?' GLENN: K-R-O-K-O-D-I-A-L'
TEACHER: No, that's wrong
GLENN: Maybe it is wrong, but you asked me how I spell it.
(I Love this child)
____________________________________
TEACHER: Donald, what is the chemical formula for water?
DONALD: H I J K L M N O.
TEACHER: What are you talking about?
DONALD: Yesterday you said it's H to O.
____________________________________
TEACHER: Winnie, name one important thing we have today that we didn't have ten years ago.
WINNIE: Me!
____________________________________
TEACHER: Glen, why do you always get so dirty?
GLEN: Well, I'm a lot closer to the ground than you are.
____________________________________
TEACHER: Millie, give me a sentence starting with ' I. '
MILLIE: I is..
TEACHER: No, Millie..... Always say, 'I am.'
MILLIE: All right... 'I am the ninth letter of the alphabet.'
____________________________________
TEACHER: George Washington not only chopped down his father's cherry tree, but also admitted it. Now, Louie, do you know why his father didn't punish him?
LOUIS: Because George still had the axe in his hand.....
____________________________________
TEACHER: Now, Simon , tell me frankly, do you say prayers before eating?
SIMON: No sir, I don't have to, my Mom is a good cook.
____________________________________
TEACHER: Clyde , your composition on 'My Dog' is exactly the same as your brother's.. Did you copy his?
CLYDE : No, sir. It's the same dog.
(I want to adopt this kid!!!)
____________________________________
TEACHER: Harold, what do you call a person who keeps on talking when people are no longer interested?
HAROLD: A teacher