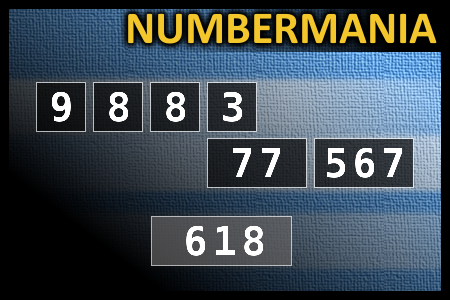
Calculate the number 618
NUMBERMANIA: Calculate the number 618 using numbers [9, 8, 8, 3, 77, 567] and basic arithmetic operations (+, -, *, /). Each of the numbers can be used only once.
Busy Bus Stop
In a crowded city at a busy bus stop, a woman who was waiting for a bus was wearing a tight leather skirt. As the bus stopped and it was her turn to get on, she became aware that her skirt was too tight to allow her leg to come up to the height of the first step of the bus. Slightly embarrassed and with a quick smile to the bus driver, she reached behind her to unzip her skirt a little, thinking that this would give her enough slack to raise her leg.
Again, she tried to make the step only to discover she still couldn't. So, a little more embarrassed, she once again reached behind her to unzip her skirt a little more. For the second time, attempted the step, and, once again, and much to her chagrin, she could not raise her leg. With a little smile to the driver, she again reached behind to unzip a little more and again was unable to make the step.
About this time, a big man who was standing behind her picked her up easily by the waist and placed her gently on the step of the bus.
She went ballistic and turned to the would-be Samaritan and screeched, "How dare you touch my body! I don't even know who you are!' The man smiled and drawled, "Well, ma'am, normally I would agree with you, but after you unzipped my fly three times, I kinda figured we was friends."