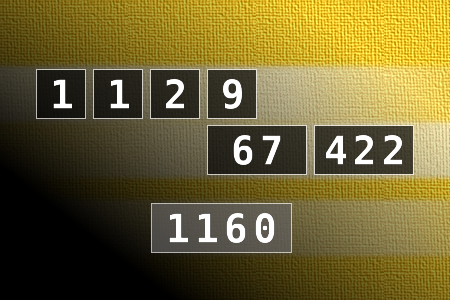Calculate the number 1160
NUMBERMANIA: Calculate the number 1160 using numbers [1, 1, 2, 9, 67, 422] and basic arithmetic operations (+, -, *, /). Each of the numbers can be used only once.Correct answers: 10
The first user who solved this task is Nasrin 24 T.
#brainteasers #math #numbermania

A guy was on trial for murder...
A guy was on trial for murder and if convicted, would get the electric chair. His brother found out that a redneck was on the jury and figured he would be the one to bribe. He told the redneck that he would be paid $10,000 if he could convince the rest of the jury to reduce the charge to manslaughter.
The jury was out an entire week and returned with a verdict of manslaughter.
After the trial, the brother went to the redneck's home, told him what a great job he had done and paid him the $10,000.
The redneck replied that it wasn't easy to convince the rest of the jury to change the charge to manslaughter. They all wanted to let him go.
The jury was out an entire week and returned with a verdict of manslaughter.
After the trial, the brother went to the redneck's home, told him what a great job he had done and paid him the $10,000.
The redneck replied that it wasn't easy to convince the rest of the jury to change the charge to manslaughter. They all wanted to let him go.