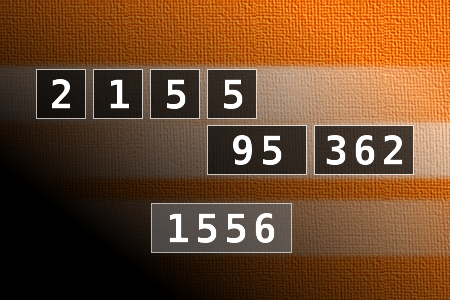Calculate the number 1556
NUMBERMANIA: Calculate the number 1556 using numbers [2, 1, 5, 5, 95, 362] and basic arithmetic operations (+, -, *, /). Each of the numbers can be used only once.Correct answers: 8
The first user who solved this task is Nasrin 24 T.
#brainteasers #math #numbermania

Jump out of the plane
An Englishman, Frenchman, Mexican, and Texan were flying across country on a small plane when the pilot comes on the loud speaker and says " We're having mechanical problems and the only way we can make it to the next airport is for 3 of you to open the door and jump, at least one of you can survive"
The four open the door and look out below. The Englishman takes a deep breath and hollers "God Save The Queen" and jumps.
The Frenchman gets really inspired and hollers "Viva La France" and he also jumps.
This really pumps up the Texan so he hollers "Remember the Alamo" and he grabs the Mexican and throws him out of the plane.