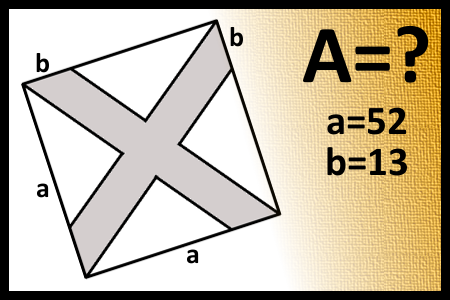
Find the area of the shaded figure
Express result to the accuracy of 3 decimal.Correct answers: 22
The first user who solved this task is Djordje Timotijevic.
#brainteasers #math

Two small boys met during thei...
Two small boys met during their first day at school. "My name is Billy. What's yours?" asked the first boy.
"Tommy," replied the second.
"My daddy is an accountant. What does your daddy do for a living?" asked Billy.
Tommy replied, "My daddy is a lawyer."
"Honest?" asked Billy.
"No, just the normal kind," replied Tommy.
"Tommy," replied the second.
"My daddy is an accountant. What does your daddy do for a living?" asked Billy.
Tommy replied, "My daddy is a lawyer."
"Honest?" asked Billy.
"No, just the normal kind," replied Tommy.