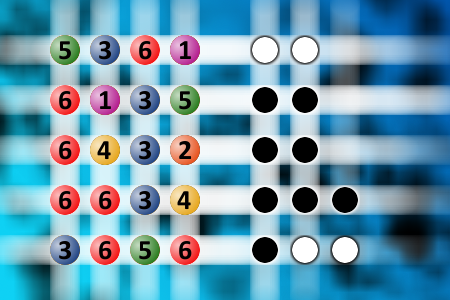
Find the right combination
The computer chose a secret code (sequence of 4 digits from 1 to 6). Your goal is to find that code. Black circles indicate the number of hits on the right spot. White circles indicate the number of hits on the wrong spot.Correct answers: 21
The first user who solved this task is Nasrin 24 T.
#brainteasers #mastermind