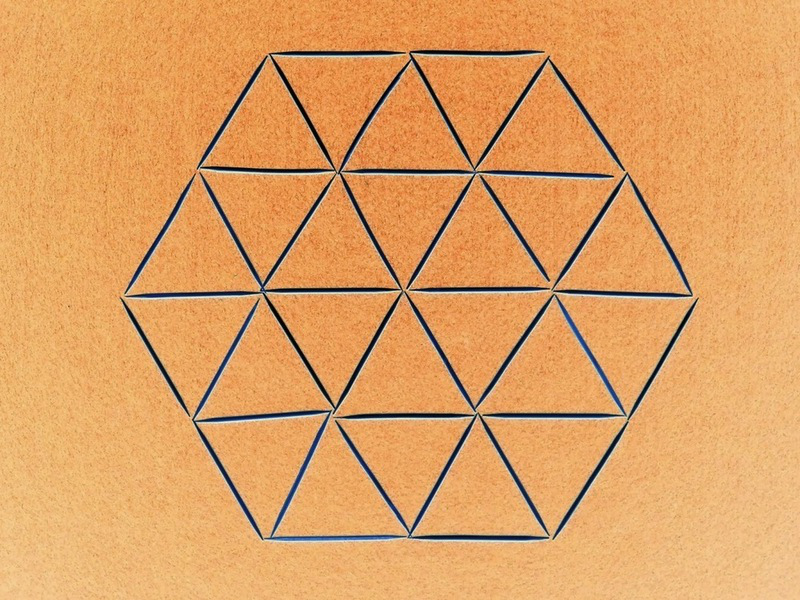
How Many Regular Hexagons?
How many regular hexagons can you count in this image?Correct answers: 144
The first user who solved this task is Djordje Timotijevic.
#brainteasers #math #riddles

Offer Legal Advice
Taylor was desperate for business, and was happy to be appointed by the court to defend an indigent defendant.
The judge ordered Taylor, "You are to confer with the defendant in the hallway, and give him the best legal advice you can."
After a time, Taylor re-entered the courtroom alone.
When the judge asked where the defendant had gone, Taylor replied, "You asked me to give him good advice. I found out that he was guilty, so I told him to split."
The judge ordered Taylor, "You are to confer with the defendant in the hallway, and give him the best legal advice you can."
After a time, Taylor re-entered the courtroom alone.
When the judge asked where the defendant had gone, Taylor replied, "You asked me to give him good advice. I found out that he was guilty, so I told him to split."