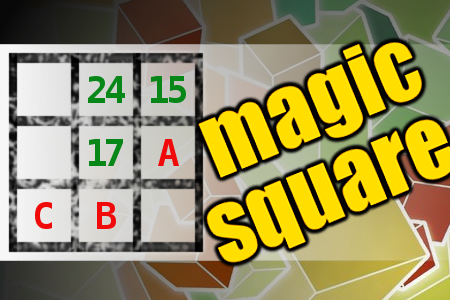
MAGIC SQUARE: Calculate A-B+C
The aim is to place the some numbers from the list (5, 6, 8, 14, 15, 17, 24, 25, 27) into the empty squares and squares marked with A, B an C. Sum of each row and column should be equal. All the numbers of the magic square must be different. Find values for A, B, and C. Solution is A-B+C.Correct answers: 11
The first user who solved this task is Nasrin 24 T.
#brainteasers #math #magicsquare

A lawyer was cross-examining t...
A lawyer was cross-examining the doctor about whether or not he had checked the pulse of the deceased before he signed the death certificate. "No," the doctor said. "I did not check his pulse." "And did you listen for a heartbeat?" asked the lawyer. "No I did not," the doctor said. "So," said the lawyer, "when you signed the death certificate, you had not taken steps to make sure he was dead." The doctor said, "Well, let me put it this way. The man's brain was in a jar on my desk but, for all I know, he could be out practicing law somewhere."