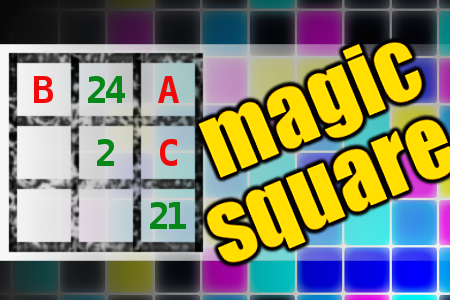
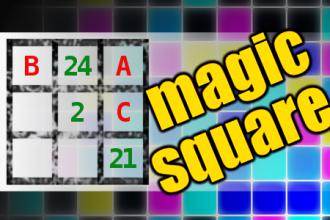
MAGIC SQUARE: Calculate A-B-C
The aim is to place the some numbers from the list (2, 5, 8, 21, 24, 27, 37, 40, 43, 98) into the empty squares and squares marked with A, B an C. Sum of each row and column should be equal. All the numbers of the magic square must be different. Find values for A, B, and C. Solution is A-B-C.
You will spend eternity here
The devil meets him at the gate and says, "Alright, you have died and come to hell. You will spend eternity here, but you get to choose how to spend it. You may choose one of these three doorways. Once you choose a door, you may not change it. So let's get started."
The devil opens Door One. The guy looks in and sees a couple of people standing on their heads on a Concrete floor. The guy says, "No way, let's move on."
The devil opens Door Two. The guy sees a few more people standing on their heads on a Wood floor. The guy says, "No way, let's move on."
The devil opens Door Three. The guy sees a bunch of people standing knee-deep in cow manure drinking coffee. The guy says, "Great, this is the one I will chose." The devil says, "OK, wait right here, I will get you some coffee."
The guy settles in with his coffee thinking that this isn't so bad. What's the big deal?
After about 10 minutes a voice comes over the loud speaker saying, "Coffee break's over. Back on your heads!"