What a winning combination?
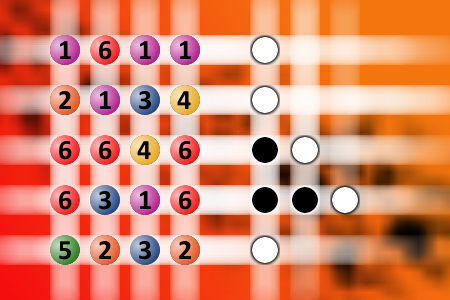
The computer chose a secret code (sequence of 4 digits from 1 to 6). Your goal is to find that code. Black circles indicate the number of hits on the right spot. White circles indicate the number of hits on the wrong spot.Correct answers: 19
The first user who solved this task is Nasrin 24 T.
#brainteasers #mastermind

Moths
A woman was having a passionate affair with an inspector from pest-control company. One afternoon they were carrying on in the bedroom together when her husband arrived home unexpectedly. "Quick," said the woman to her lover, "into the closet!", and she pushed him into the closet stark naked. The husband, however, became suspicious and after a search of the bedroom discovered the man in the closet. "Who are you?" he asked him.
"I'm an inspector from Bugs-B-Gone," said the exterminator.
What are you doing in there?" the husband asked.
I'm investigating a complaint about an infestation of moths," the man replied.
"And where are your clothes?" asked the husband.
The man looked down at himself and said, "Those little bastards!"