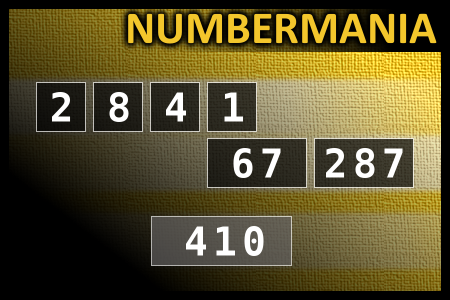
Calculate the number 410
NUMBERMANIA: Calculate the number 410 using numbers [2, 8, 4, 1, 67, 287] and basic arithmetic operations (+, -, *, /). Each of the numbers can be used only once.Correct answers: 44
The first user who solved this task is Sanja Šabović.
#brainteasers #math #numbermania

Mommy, my turtle is dead...
"Mommy, my turtle is dead," the little boy, Freddie, sorrowfully told his mother, holding the turtle out to her.
The mother kissed him on the head, then said, "That's all right. We'll wrap him in tissue paper, put him in a little box, then have a nice burial ceremony in the back yard. After that, we'll go out for an ice cream soda, and then get you a new pet. I don't want you...." Her voice trailed off as she noticed the turtle move.
"Freddie, your turtle is not dead after all."
"Oh," the disappointed boy said. "Can I kill it?"
The mother kissed him on the head, then said, "That's all right. We'll wrap him in tissue paper, put him in a little box, then have a nice burial ceremony in the back yard. After that, we'll go out for an ice cream soda, and then get you a new pet. I don't want you...." Her voice trailed off as she noticed the turtle move.
"Freddie, your turtle is not dead after all."
"Oh," the disappointed boy said. "Can I kill it?"