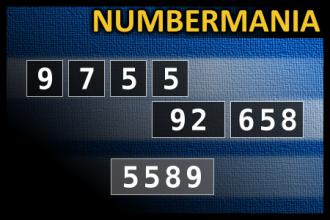
Calculate the number 5589
NUMBERMANIA: Calculate the number 5589 using numbers [9, 7, 5, 5, 92, 658] and basic arithmetic operations (+, -, *, /). Each of the numbers can be used only once.Correct answers: 22
The first user who solved this task is Sanja Šabović.
#brainteasers #math #numbermania