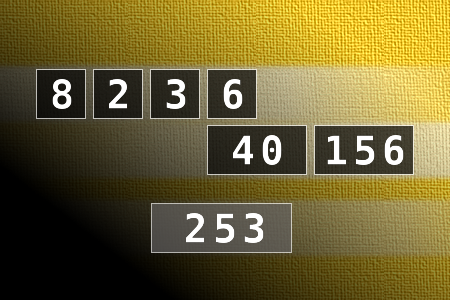Calculate the number 253
NUMBERMANIA: Calculate the number 253 using numbers [8, 2, 3, 6, 40, 156] and basic arithmetic operations (+, -, *, /). Each of the numbers can be used only once.
Bill & Moe
Bill and Moe had started with only five hundred dollars between them, but they had built up a computer business with sales in the millions. Their company employed over two hundred people, and the two executives lived like princes.
Almost overnight, things changed. Sales dropped sharply, former customers disappeared, the business failed, and personal debts forced both into bankruptcy. Bill and Moe blamed each other for the troubles, and they parted on unfriendly terms.
Five years later, Bill drove up to a decrepit diner and stopped for a cup of coffee. As he was discreetly wiping some crumbs from the table, a waiter approached. Bill looked up and gasped.
"Moe!" he said, shaking his head. "It's a terrible thing, seeing you working in a place as bad as this."
"Yeah," Moe said with a smirk. "But at least I don't eat here."