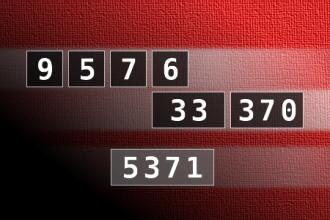
Calculate the number 5371
NUMBERMANIA: Calculate the number 5371 using numbers [9, 5, 7, 6, 33, 370] and basic arithmetic operations (+, -, *, /). Each of the numbers can be used only once.Correct answers: 1
#brainteasers #math #numbermania

Couple at the cinema
One day, a man was dragged to the cinema by his wife who wanted to watch a romantic comedy.
Half an hour into the film, the man felt a nudge in his elbow. "What an outrage," his wife murmured to him.
"The person sitting in front of us is sleeping!" the woman said, clearly offended.
Her husband was fairly annoyed.
He replied: "You woke me up to tell me that?"