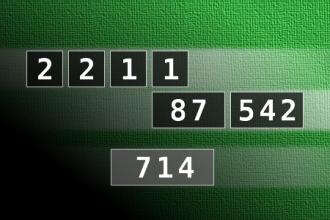
Calculate the number 714
NUMBERMANIA: Calculate the number 714 using numbers [2, 2, 1, 1, 87, 542] and basic arithmetic operations (+, -, *, /). Each of the numbers can be used only once.Correct answers: 1
#brainteasers #math #numbermania

A woman was out golfing one da...
A woman was out golfing one day when she hit her ball into the woods. She went into the woods to look for it and found a frog in a trap.
The frog said to her, "If you release me from this trap, I will grant you 3 wishes."
The woman freed the frog and the frog said, "Thank you, but I failed to mention that there was a condition to your wishes - that whatever you wish for, your husband will get 10 times more or better!"
The woman said, "That would be okay," and for her first wish, she wanted to be the most beautiful woman in the world.
The frog warrned her, "You do realize that this wish will also make your husband the most handsome man in the world, an Adonis, that women will flock to."
The woman replied, "That will be okay because I will be the most beautiful woman and he will only have eyes for me."
So, KAZAM - she's the most beautiful woman in the world!
For her second wish, she wanted to be the richest woman in the world.
The frog said, "That will make your husband the richest man in the world, and he will be ten times richer than you."
The woman said, "That will be okay because what is mine is his and what is his is mine."
So, KAZAM she's the richest woman in the world!
The frog inquired about her third wish, and she answered, "I'd like a mild heart attack."
The frog said to her, "If you release me from this trap, I will grant you 3 wishes."
The woman freed the frog and the frog said, "Thank you, but I failed to mention that there was a condition to your wishes - that whatever you wish for, your husband will get 10 times more or better!"
The woman said, "That would be okay," and for her first wish, she wanted to be the most beautiful woman in the world.
The frog warrned her, "You do realize that this wish will also make your husband the most handsome man in the world, an Adonis, that women will flock to."
The woman replied, "That will be okay because I will be the most beautiful woman and he will only have eyes for me."
So, KAZAM - she's the most beautiful woman in the world!
For her second wish, she wanted to be the richest woman in the world.
The frog said, "That will make your husband the richest man in the world, and he will be ten times richer than you."
The woman said, "That will be okay because what is mine is his and what is his is mine."
So, KAZAM she's the richest woman in the world!
The frog inquired about her third wish, and she answered, "I'd like a mild heart attack."