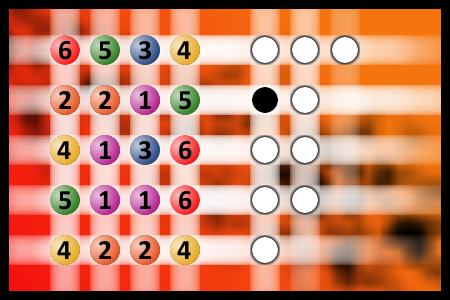
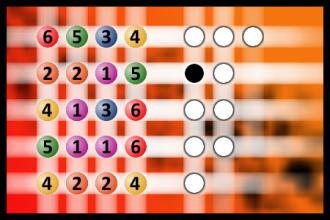
Which is a winning combination of digits?
The computer chose a secret code (sequence of 4 digits from 1 to 6). Your goal is to find that code. Black circles indicate the number of hits on the right spot. White circles indicate the number of hits on the wrong spot.Correct answers: 50
The first user who solved this task is James Lillard.
#brainteasers #mastermind

Rosebuds
The teenage granddaughter comes downstairs for her date with this see-through blouse on and no bra. Her grandmother just has a fit, telling her not to dare go out like that.
The teenager tells her "Loosen up Grams. These are modern times. You gotta let your rosebuds show!" and out she goes.
The next day the teenager comes downstairs, and the grandmother is sitting there with no top on. The teenager wants to die.
She explains to her grandmother that she has friends coming over and that it is just not appropriate.
"Loosen up, sweetie. If you can show off your rosebuds, then I can display my hanging baskets."