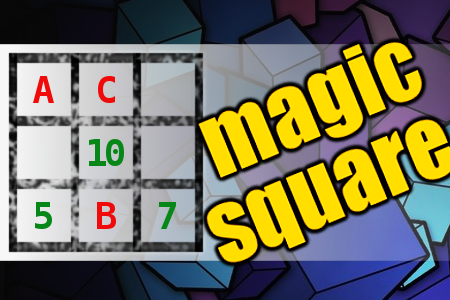
MAGIC SQUARE: Calculate A-B+C
The aim is to place the some numbers from the list (2, 5, 7, 10, 13, 16, 34, 39, 45, 47) into the empty squares and squares marked with A, B an C. Sum of each row and column should be equal. All the numbers of the magic square must be different. Find values for A, B, and C. Solution is A-B+C.Correct answers: 30
The first user who solved this task is Djordje Timotijevic.
#brainteasers #math #magicsquare

Six guys were playing poker wh...
Six guys were playing poker when Smith loses $500 on a single hand, clutches his chest and drops dead at the table. Showing respect for their fallen comrade, the other five complete their playing time standing up. Roberts looks around and asks, "Now, who is going to tell the wife?" They draw straws. Rippington, who is always a loser, picks the short one. They tell him to be discreet, be gentle, don't make a bad situation any worse than it is. "Gentlemen! Discreet? I'm the most discreet man you will ever meet. Discretion is my middle name, leave it to me." Rippington walks over to the Smith house, knocks on the door, the wife answers, and asks what he wants. Rippington says, "Your husband just lost $500 playing cards." She hollers, "TELL HIM TO DROP DEAD!" Rippington says, "I'll tell him.