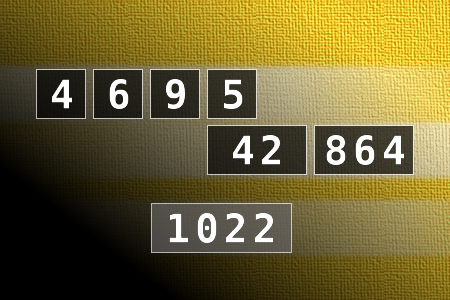Calculate the number 1022
NUMBERMANIA: Calculate the number 1022 using numbers [4, 6, 9, 5, 42, 864] and basic arithmetic operations (+, -, *, /). Each of the numbers can be used only once.Correct answers: 1
#brainteasers #math #numbermania

A man is at the airport counter checking in his luggage...
A man is at the airport counter checking in his luggage.
The man said to the agent, "I'm flying to Los Angeles but I would like this bag to go to Portland, this one to Albuquerque, and this one to Sioux Falls."
The agent looked suitably shocked and said, "Sir, there is no way we can do that."
"Why not?", replied the man, "You did it last time".